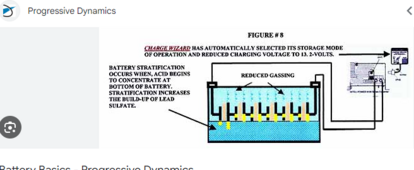
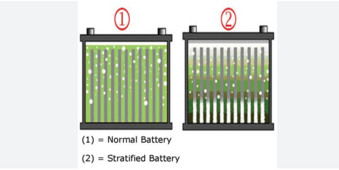
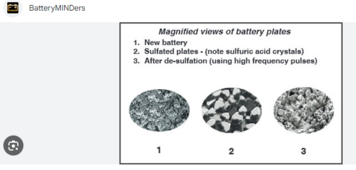
I've read this new (20230 study about desulfating flooded lead acid batteries with just high voltage DC charge. It is very interesting as they tested just overvoltage on desulfating flooded lead acid. Many a company sells pulse desulfators, but if all we need is a bench power supply - that would be a great discovery.
They did a proper study with multiple batches of 30 batteries and they have microscope photos etc to prove it. So it seems legit. The study is: "A Novel Approach Using High Charging Voltage for the Restoration of Discarded Lead Acid Batteries" by Chee Hiun Lee, Jianhiu Wong, Yun, Seng Lin. It is available here: https://www.researchgate.net/public..._Restoration_of_Discarded_Lead_Acid_Batteries
the conclusion of the study is that we can desulfate and achieve close to 80% of capacity without the "inverse charge", we just apply overvoltage of 2.6V per cell. They tested with 2.67V and found it works too, but it removes too much electrolyte. 2.6V seems to be enough to desulfate without (too many) adverse effects.
2.6V per cell is 15.6V for a 12V battery. They did all their tests with tiny 7Ah discarded UPS batteries and the gist of it is to pump 15.6V(for a 12V battery) for 24h into it, then charge normally for 48h and its done. The current should slowly raise up till 0.5A (for a 7Ah battery). Of course they had a number of failures, all were caused by mechanical deformation of conductors causing shorts or disconnects.
I have 4 198Ah 6V batteries (for a total of 24V) which were left with no maintenance for 6 months in temperatures around 2C (they were fully charged before). All 4 show slight whitening of the plates. 3 sit at 6.3V one has collapsed to 5V. The one that was collapsed was charged fully and the capacity was tested at 1Ah only!
Then I tried 3h of this overvoltage treatment on the worst battery. And indeed the current raised up to about 4A. I then tested the battery and it tested as 19Ah. Promising, but far from the result I hoped. So I gave it additional 8h and I then noticed the remaining 3 batteries are sulfated too. So I connected them all 4 in a string and I gave them 30.2V from a constant current PSU limited to 10A. It has been about 8h more and the batteries are starting to get slightly warm. They are of course bubbling a lot and the plates look the correct color.
10A seems much, but considering they used 0.5A with a battery almost 20 times snmallert (and they do say it reached 45C temperature in the end) I think I'm OK. I'm planing to keep going to complete the 24h, then do a "normal charge" for 48h and do a capacity test.
Has anyone done desulfation of good, but sulfated batteries before? Also, in case anyone is interested, these appear to be lead-calcium alloy batteries. This does matter for the voltages involved. Sadly the researchers didn't specify what alloy of lead they did their experiments with. Perhaps I need to write to them to ask
They did a proper study with multiple batches of 30 batteries and they have microscope photos etc to prove it. So it seems legit. The study is: "A Novel Approach Using High Charging Voltage for the Restoration of Discarded Lead Acid Batteries" by Chee Hiun Lee, Jianhiu Wong, Yun, Seng Lin. It is available here: https://www.researchgate.net/public..._Restoration_of_Discarded_Lead_Acid_Batteries
the conclusion of the study is that we can desulfate and achieve close to 80% of capacity without the "inverse charge", we just apply overvoltage of 2.6V per cell. They tested with 2.67V and found it works too, but it removes too much electrolyte. 2.6V seems to be enough to desulfate without (too many) adverse effects.
2.6V per cell is 15.6V for a 12V battery. They did all their tests with tiny 7Ah discarded UPS batteries and the gist of it is to pump 15.6V(for a 12V battery) for 24h into it, then charge normally for 48h and its done. The current should slowly raise up till 0.5A (for a 7Ah battery). Of course they had a number of failures, all were caused by mechanical deformation of conductors causing shorts or disconnects.
I have 4 198Ah 6V batteries (for a total of 24V) which were left with no maintenance for 6 months in temperatures around 2C (they were fully charged before). All 4 show slight whitening of the plates. 3 sit at 6.3V one has collapsed to 5V. The one that was collapsed was charged fully and the capacity was tested at 1Ah only!
Then I tried 3h of this overvoltage treatment on the worst battery. And indeed the current raised up to about 4A. I then tested the battery and it tested as 19Ah. Promising, but far from the result I hoped. So I gave it additional 8h and I then noticed the remaining 3 batteries are sulfated too. So I connected them all 4 in a string and I gave them 30.2V from a constant current PSU limited to 10A. It has been about 8h more and the batteries are starting to get slightly warm. They are of course bubbling a lot and the plates look the correct color.
10A seems much, but considering they used 0.5A with a battery almost 20 times snmallert (and they do say it reached 45C temperature in the end) I think I'm OK. I'm planing to keep going to complete the 24h, then do a "normal charge" for 48h and do a capacity test.
Has anyone done desulfation of good, but sulfated batteries before? Also, in case anyone is interested, these appear to be lead-calcium alloy batteries. This does matter for the voltages involved. Sadly the researchers didn't specify what alloy of lead they did their experiments with. Perhaps I need to write to them to ask